What Is the Most Useful Property of the Semimetals
Metalloids share characteristics of both metals and non-metals and are also called semimetals. What is the most useful property of metalloids.

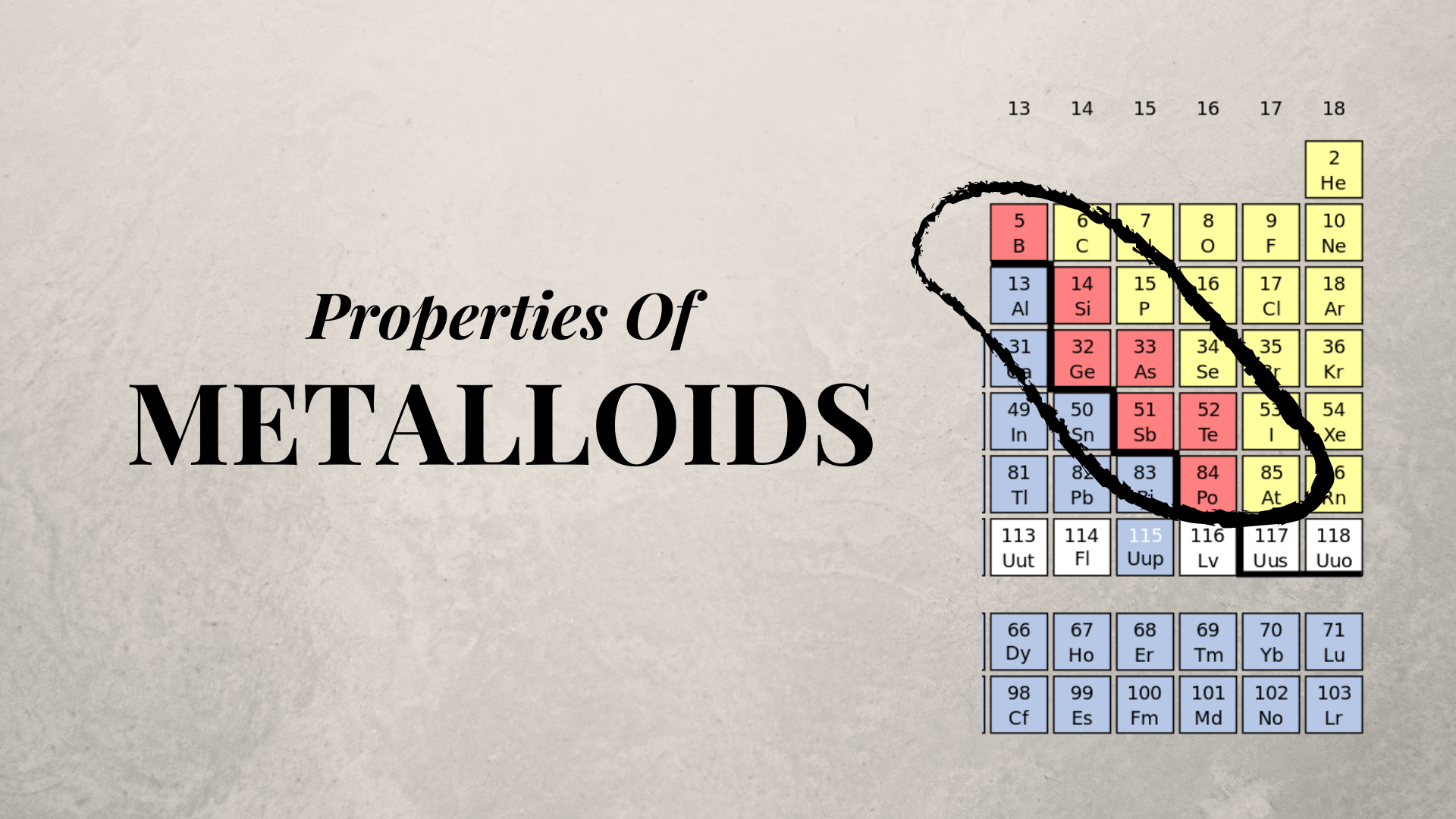
Metalloids The Semi Metals In The Periodic Table
To make most synthetic elements scientists use powerful machines called _____.

. Could be dull or shiny. The classic semimetallic elements are arsenic antimony bismuth α-tin gray tin and graphite an allotrope of carbon. Some important examples of metalloids are as follows.
The most useful property of the metalloids is their varying ability to conduct electricity. This semi-conducting property makes Metalloids. What are three properties of metalloids or semimetals.
Ability to bond b. Is magnesium a semi metal. Metalloids may act either like metals or nonmetals in chemical reactions.
What is the most useful property of semi-metals. What is a semiconductor. Whether or not a metalloid conducts electricity can depend on the temperature or the exposure to light.
The concept of the Fermi surface is very useful in condensed matter physics for predicting the thermal electrical magnetic and optical properties of metals semimetals and doped semiconductorsThe Fermi surface is an abstract boundary in a reciprocal space and its. Also what are the most common metalloids. Whats another name for Semimetal.
They are also called as semi metals. Metalloids Metalloids are sometimes called semimetals a practice that has been discouraged as the term semimetal has a different meaning in physics than in chemistry. Metalloids often have the following properties.
Up to 24 cash back called semimetals. Hereof what is the most useful property of the Semimetals. Can both gain and lose electrons in reactions.
What information in the periodic table indicates the number of protons in an atom. The most useful property of the semimetals is their varying ability to conduct electric current. The elements atomic number.
Semiconductors are substances that under some conditions can carry. Log in for more information. What are some examples of semi metals.
Ability to form alloys with metals. 1 Show answers Another question on Physics. Conducts heat and electricity but not as well as metals.
The reactivity of metalloids depends on the properties of the elements they are interacting with. Pearson Chemistry Matta Staley Waterman Wilbraham. Related Questions What are the three semi metals.
Asked 262015 81020 AM. Often having a metallic luster although they may have allotropes that appear nonmetallic. Roy in Materials Under Extreme Conditions 2017 311 Orbiting Electrons and Quantum Oscillations.
The reactivity of metals tends to decrease as you move from left to right across the periodic table. Updated 262015 82913 AM. Most metalloids have a shiny metallic appearance but are brittle unexceptional electrical conductors and display nonmetallic chemical properties.
The semimetals have some of the characteristics of metals and some of the characteristics of nonmetals. What property of semi-metals is useful in the computer industry. The most useful property of semimetalsmetalloids.
Highly reactive Semi-conducting is useful in the computer industry. TSMs have attracted broad interests for both the fundamental research. The most useful property of the semimetals is their varying ability to conduct electric currents.
Positive ions differ from neutral atoms in that. Metalloids are typically semi-conductors which means that they both insulate and conduct electricity. Explain what makes a passenger in a turning car slide toward the door.
The most useful property of the metalloids is their varying ability to conduct electricity. Liquid at room temperature. Varying ability to transfer heat and electricity 100.
The most useful property of metalloids is their varying ability to conduct electricity. Between the metals and nonmetals is a group of elements known as either the semimetals or the metalloids which are elements that have properties intermediate between those of the metals and nonmetals. Metalloids tend to be good semiconductors.
The most useful property of the metalloids is their varying ability to conduct electricity. Elements closer to the noble gases have. The semiconductor property makes metalloid very useful as a computer chip material.
Layered topological semimetals TSMs as new states of quantum matter have been regarded as one of the most intriguing materials with strong spinorbit coupling SOC nontrivial band topology and unusual features of monolayer. For this reason metalloids such as silicon or germanium are used to make semiconductors. The most useful property of the semimetals is their varying ability to conduct electric current.
Which property of bromine could you NOT predict based on the fact that it is a nonmetal in the halogen family. What is the most useful property of semimetals. The most useful property of the semimetals is their varying ability to conduct electric current.
Xuefeng Wang Minhao Zhang in Spintronic 2D Materials 2020. The most useful property of semimetals is their. Varying ability to conduct electric current.
Usually behaving as nonmetals in chemical reactions. Metalloids may have a metallic luster but they also have our tropes which can have a nonmetallic appearance. In a chemical reaction the properties of the reactants and the properties of the products are _____ What is different.
BoronB SiliconSi and GermaniumGe. What is the most useful property of Semimetals. Semimetalsmetalloids have some characteristics of nonmetals and some characteristics of metals.
Some semimetals are used to make semiconductors. What are three uses of Semimetals.
4 Properties Of Metalloids Science Trends

Notes 4 3 And 4 4 Metals Nonmetals Inert Gases And Semimetals Ppt Download
Comments
Post a Comment